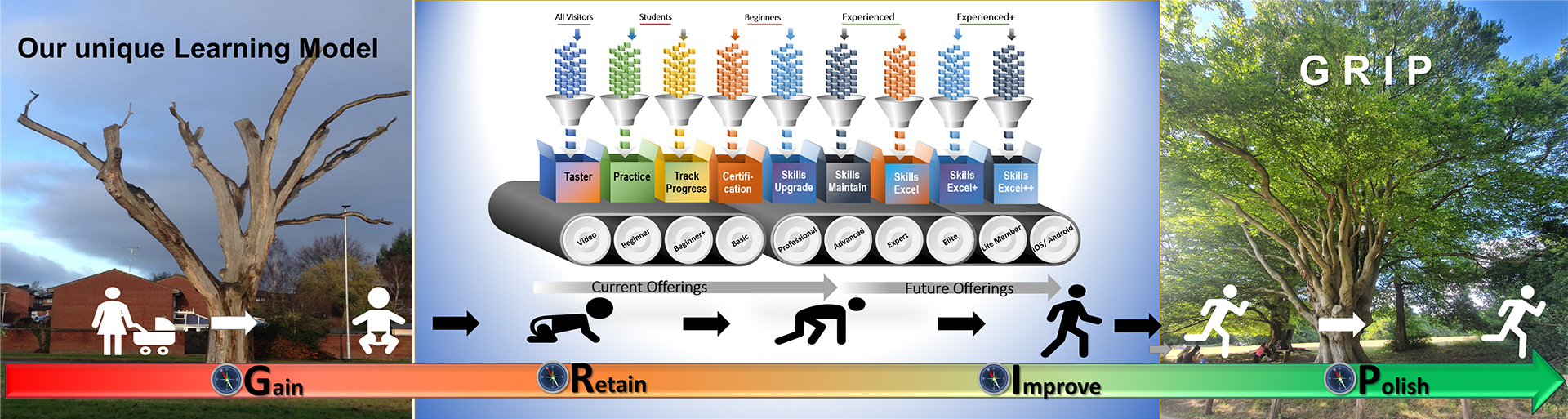
The New Way to Success Advance Progress
Review ongoing regulatory guidances/ changes and then propose some customised solutions to protect patient safety, data integrity and to promote a high-quality clinical research conduct.

How GCP COMPASS may help in the future ?
We all have experienced the impact of COVID-19 crisis and while there are global efforts to get a control over this and get personal lives and business moving, we need to be prepared for such crisis in the future. Now it is COVID-19 and tomorrow; it may be something else and that may mean; another outbreak of similar nature or may be another pandemic, or even a natural disaster (earthquake, wild fire, storm/ tsunami) and the list may go endless.
Although we can learn from our past experiences, the challenges such calamities will bring are not always likely to be the same and this is where we need to see how one can modernise clinical research by ensuring global requirements and more importantly respecting regional/ local considerations to deliver proactive quality. GCP COMPASS aims to make its own contribution to such efforts.
Our dynamic GCP COMPASS Virtual Learning Environment (VLE) Platform and future offerings will ensure we help you stay connected and focused to understand & implement ongoing changes in clinical research rules, regulations and guidelines effectively.
